On the continuous relevance of “naked nickel” in sustainable catalysis
Celebrating the 100th Birthday of Prof. Günther Wilke
The nickel catalysis work of Prof. Wilke still inspires new developments in sustainable catalysis at the Kohlenforschung like ligand-free Suzuki–Miyaura couplings.
This year marks the 100th birthday of the former Director of the Max-Planck-Institut für Kohlenforschung, Prof. Günther Wilke (1925–2016), a visionary chemist whose work shaped the field of organonickel chemistry. Born on February 23, 1925, in Heidelberg, Germany, Wilke began his scientific journey under the guidance of Prof. Karl Freudenberg, completing his doctoral studies in 1951 on “Untersuchungen über die Formaldehyd abspaltende Gruppe im Lignin und in Modellsubstanzen”. After his graduation in 1951 he joined the Max-Planck-Institut für Kohlenforschung as scientific assistant of Prof. Karl Ziegler, where he quickly distinguished himself as an exceptional scientist. Among his early achievements were his invaluable contributions to the cyclotrimerization of butadiene to cyclododeca-1,5,9-triene with titanium and nickel based Ziegler-catalysts. These developments had major implications in industrial production, as exemplified by the multi thousand-ton scale process for the production of nylon derivatives such as Vestamide, the polymeric material present in West Germany’s football shoes that won the World Cup in 1974.
It was however, Wilke’s pioneering work on the chemistry of “naked nickel” complexes that earned him lasting recognition as the father of modern Ni-olefin chemistry. In 1962, during mechanistic investigations on the nickel-effect in the oligomerization of butadiene, he isolated the highly sensitive Ni(0) complexes Ni(COD)₂ and Ni(t‐CDT). The former complex remains still today the “go to” source of Ni(0) in organonickel chemistry, being a widely used pre-catalyst by many researchers. The rapid adoption of Ni(COD)2 by the community is due to its incredible properties as pre-catalyst: Ni(COD)2 has a tremendous propensity to exchange its olefinic ligands in favor of other more coordinating ligands. Hence, Prof. Wilke described these complexes as “naked nickel” complexes — a concept that revolutionized transition metal complex synthesis and catalysis. Following these seminal reports, a myriad of examples followed by Prof. Wilke and his team at The Kohlenforschung, providing in depth analysis of this phenomena. These efforts led to the development of a plethora of novel Ni(0)-olefin complexes and Ni-catalyzed processes, thus pushing the boundaries of knowledge for future generations to come. Nowadays his legacy continues to be present in both academic and industrial settings, with Ni(COD)2 dominating as the most employed Ni-source.
Almost 60 years after the seminal work of Prof. Wilke on “naked nickel” Ni(COD)2, the group of Dr. Josep Cornella at the MPI KOFO developed a series of air-stable (Ni(Rstb)3) pre-catalysts. The development of these complexes is considered a milestone in the field of “naked nickel” chemistry, thus permitting practitioners in the field to set up reactions with Ni(0) without the need of excluding air during manipulation and handling. These pre-catalysts have been commercialized and already incorporated into every chemists’ toolbox, as exemplified by the recent synthetic applications by the group of Prof. Alois Fürstner, Director of the Department of Organometallic Chemistry at The Kohlenforschung.
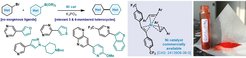
Inspired by the seminal work of Prof. Wilke on the concept of “naked nickel”, a team of researchers led by Dr. Cornella developed a catalytic protocol for the formation of heteroaryl-heteroaryl bonds. Using the air-stable Ni(Rstb)3 catalysts platform, the team unlocked an unprecedented ligand-free Suzuki-Miyaura coupling between heteroaryl boronic acids and heteroaryl bromides. Such catalytic formation through Ni catalyzed processes is challenging, with limited protocols reported to date. Yet, the development of this catalytic method permits access to a wide scope of heterobiaryls, including biologically relevant and medicinally crucial compounds. Beyond the applicability, the protocol represents a paradigm shift in catalyst design for couplings: the catalytic system is characterized by the absence of σ-donating ligands, commonly employed and required for efficient C‒C bond formation. This novel approach to cross-coupling is the vivid demonstration of Prof. Wilke’s legacy. The “naked nickel” concept he coined more than 65 years ago is still very much alive and crucial at providing solutions to current problems in chemical research, thus demonstrating the lasting impact of his discoveries. Indeed, Wilke’s influence on modern catalysis cannot be overstated and his footsteps are still followed by many, not only at the The Kohlenforschung but also to the myriad of minds he continues to inspire.
Recent Publication
Authors: Rakan Saeb & Josep Cornella













