Directed Evolution of Enzymes as Catalysts in Synthetic Organic Chemistry

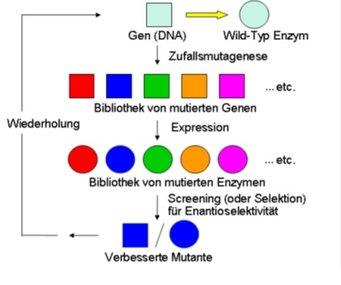
Enzymes have been used as catalysts in synthetic organic chemistry for more than 100 years, but broad application has never been achieved due to a number of traditional limitations including the often observed narrow substrate scope, poor stereoselectivity and/or insufficient stability. The molecular biological methods of directed evolution offer a way out of this dilemma. Some time ago we introduced a fundamentally new approach to asymmetric catalysis, namely directed evolution of stereoselective enzymes as catalysts in synthetic organic chemistry and biotechnology. In more recent times methodology development has become crucial, aimed at making directed evolution in general more efficient, faster and reliable. Especially iterative saturation mutagenesis (ISM) as developed in our lab has proven to be highly successful, allowing problems associated with limited substrate scope, poor or wrong stereo- and/or regioselectivity as well as thermostability to be solved within a short time. We generally focus on stereoselective transformations which are not possible with current synthetic catalysts, e.g., regio- and stereoselective oxidative hydroxylation of simple and complex organic compounds. Our methods are being used in pharmaceutical and biotechnological industries for ecologically and economically viable transformations.