Computational Chemistry

Our computational chemistry applications center around the reactions depicted above. Areas of recent interest are centered around:
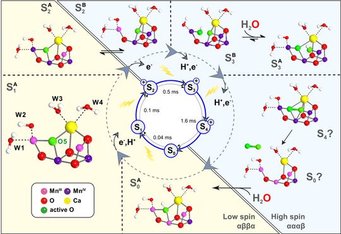
(a) The oxidation of water by the oxygen evolving complex (OEC) of Photosystem II (PSII). This research area is led by Dr. Dimitrios Pantazis and is carried out in close collaboration with the department of Prof. Wolfgang Lubitz. The efforts that have led to the proposal of a refined structure for the OEC that is consistent with all crystallographic and spectroscopic data.[2, 19] Our desire to understand the reaction mechanism of the OEC on the basis of its spectroscopic properties[2, 19a, 19e] has led us to consider the properties of manganese complexes in greater detail and has led to a series of systematic investigations on manganese monomers, dimers and oligomers e.g.[10c, 11b, 20] Recent reviews summarize the state of affairs.[19c, 21]
Figure 9
Fig. 9: A combination of X-ray emission and quantum chemistry reveals that the active site of nitrogenase contains a central carbide ion.
(b) The activation of dinitrogen, one of the most inert molecules known in chemistry, by the enzyme nitrogenase is another focus of research in the group. This research area is headed by Prof. Serena DeBeer.[22] Despite intense research efforts, even the structural basis for biological nitrogen fixation has been proven elusive. Highlights include the identification of the central atom in the active site of nitrogenase to be a carbide through the combination of X–ray emission spectroscopy with quantum chemistry,[22f] the assignment of the molybdenum oxidation state as Mo(III)[22e] as well as the characterization of a nitrogen activating trinuclear iron complex (in collaboration with the group of Prof. Patrick Holland, Rochester, USA).[23]
Figure 10
Fig. 10 Left: the molecular orbitals of CO2 in linear and bent configurations. Middle: The total energy and the orbital energies of CO2 along the bending mode showing that if CO2 can be bent a low energy π* acceptor orbital becomes available as electron acceptor. Right: Correlation between the H2 splitting barrier and the hydricity of coordinated CO2 in CO2 hydrogenation reactions.
(c) The activation of CO2, another extremely inert molecule is one of the most important reactions in energy research. Conversion of CO2 to alcohols or other energy rich molecules could solve CO2 pollution problems and provide liquid fuels at the same time. Our research in this area is headed by Dr. Shengfa Ye.[24]
Figure 11
Fig. 11: The electronic structure analysis of the C-H bond activation catalyzed by high-valent iron(IV)-oxo species reveals that en route to the transition state an oxyl radical is formed that acts as a strong electrophile capable of attacking the C-H bond.
(d) The spectroscopy and reactivity of high-valent iron centers in iron enzymes and low-molecular weight catalysts. These research efforts are coordinated by Dr. Eckhard Bill (spectroscopy) and Dr. Shengfa Ye (theory).[25] A special focus of the DeBeer group is the study of the reaction mechanism of the important enzyme Methane Monooxygenase that features a dinuclear iron active site and catalyzes the chemically extremely complex transformation from methane to methanol. Highlights include the characterization of Fe(V)[26] and Fe(VI)[27] complexes (in collaboration with the former director, Prof. Karl Wieghardt), the detailed analysis of C-H bond activation reactions[28],[25a, 25e, 29] and the fascinatingly complex chemistry of iron-nitrosyls.[30]
(e) Molecular magnetism, is a fascinating research field that has been a long term interest of the department. The ultimate goal is the design of molecules (SMMs) that show magnetic hysteresis at elevated temperatures (ideally room temperature). While this goal has been proven elusive so far, important progress has been made. Importantly, after it has been realized that big oligonuclear clusters are not necessary to design molecular magnets,[8a, 17, 20a, 25f, 31] focus has shifted towards systems with only one or two transition metal ion and fascinating progress has been made towards high-temperature SMMs.86-98 Our contributions to the field range from the development of electronic structure methods to high-level applications using multireference electronic structure theory. Importantly, we have developed the method of ‘Ab initio ligand field theory’[8a, 16] that lets us deduce the classical ligand field parameters uniquely from multireference wavefunction calculations. This is invaluable for defining magnetostructural correlations and obtaining qualitative insights into the investigated systems (transition metals, lanthanides or actinides).[17, 31b, 31c, 31h, 31i]
Figure 12
Fig. 12: Ligand field theory serves as the language that connects geometric structure, electronic structure, molecular properties and reactivity. Ab initio ligand field theory (AILFT) provides a unique link between modern high-accuracy multireference electroic structure theory and LFT, thus linking computations to chemical concepts).
Figure 13
Fig. 13: Magneto-structural correlations for [Co(S-Ph)4]2- the first mononuclear complex that was reported to posses single molecule magnet properties. Left: Structure of the complex. Middle definition of the two angles theta and psi that describe the distortion of the complex from perfect tetrahedral. Right: Variation of the total energy and the zero-field splitting as a function of these two angles demonstrating two equi-energetic minima separated by a low barrier. In one minimum the complex features a small positive ZFS, in the other a large negative ZFS thus showing that minor perturbations such as second sphere effects excerted by the counter ions can lock the system into one or the other minimum).
(f) Heterogeneous catalysis - We have shown that accurate wavefunction based methods can be applied to solids and surfaces without explicitly introducing periodic boundary conditions. While this approach is limited, it is also very powerful since with present day electronic structure know-how sufficiently large clusters can be treated such that cluster model is properly approaching the properties of the bulk system. This is demonstrated in Figure 14 by showing that a) cluster calculations at the DFT level approach the results of truly periodic cluster calculations and b) that DLPNO-CCSD(T) calculations converge with respect to cluster size.[32] Once carefully extrapolated to the basis set limit these DLPNO-CCSD(T) calculations were the first to predict binding energies to surfaces with an accuracy of 1 kcal/mol.[32] However, these studies are not limited to small molecule binding to surfaces. In collaboration with the department of Prof. Schlögl, we have shown that the same strategy of correlating calculations to spectroscopy and ultimately to reactivity that is so successful in the molecular realm, can be applied as well to heterogeneous catalysts thus opening fascinating avenues for future explorations in this important field.[33]