Homogeneous Catalysis and Reaction Design
Bill Morandi joined the ETH Zurich in 2018 as Professor of Organic Chemistry. Information about his current research can be found here. This website documents the activities of the group at the Max-Planck-Institut für Kohlenforschung (until 2018).
The “homogeneous catalysis and reaction design” group has a fundamental interest in developing innovative synthetic methodologies for applications across the molecular sciences, with a particular interest in establishing new concepts in catalysis. An interdisciplinary approach drawing from the areas of organic synthesis, organometallic chemistry, supramolecular chemistry, medicinal chemistry and inorganic chemistry will be used to rationally design innovative catalytic solutions to major synthetic challenges.
Research Topics:
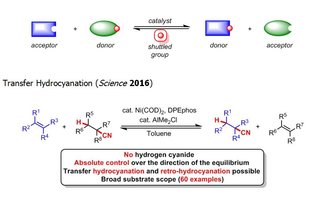
Catalytic reversible reactions, such as alkene metathesis and transfer hydrogenation, have had an auspicious impact on the molecular sciences. We are currently developing “shuttle catalysis” reactions that parallel the mechanism of transfer hydrogenation through the reversible transfer of chemical moieties beyond hydrogen, to address synthetically relevant challenges in catalysis and provide new disconnections for synthetic chemists.
[more]
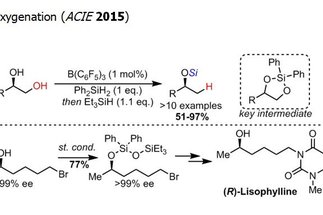
The alcohol group is one the most widespread functional groups in organic synthesis. Additionally, alcohols are ubiquitous in renewable feedstocks, such as carbohydrate derivatives. Therefore, methods for the transformation and functionalization of alcohols are in high demand in synthesis. The research area “aliphatic C–O bond activation” targets the development of novel transformations employing alcohol derivatives as starting materials, with a particular emphasis on the selective functionalization of polyol derivatives.
[more]
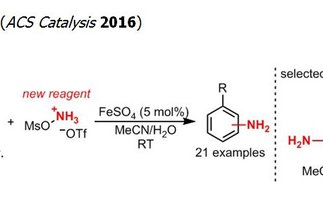
The formation of C–N bonds is one of the major challenges in the preparation of bioactive molecules. The direct catalytic amination of hydrocarbons is an attractive approach to address this challenge and has been the subject of intense research efforts. However, most of the methods developed thus far lead to the installation of a protected form of the versatile primary amine group, requiring additional and often challenging protecting group manipulations. In contrast, the research program “Direct Catalytic Synthesis of Unprotected Amines” circumvents the protecting group limitation by enabling a direct access to the desired primary amine. Besides its synthetic potential, this project also addresses a fundamental challenge in catalysis, namely the synthesis of unprotected functionalized molecules that are prone to deactivating coordination of metal catalysts.
[more]
Research Reports:
Links:
Website of Bill Morandi:
Morandilab
Follow the Morandi Lab on Twitter!
@morandilab